Best Sellers
To treat what
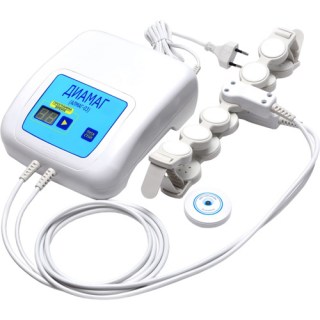
Diamag (Almag-03)
DIAMAG ( Almag 03) - Transcranial magnetic stimulation (TMS) is a noninvasive procedure that use magnetic fields to stimulate nerve cells in the brain. The therapeutic effect of the ALMAG 03 due to the use of a pulsed magnetic field. The effectiveness of techniques are proven by clinical studies. Use of this device requires no special medical education. Diamag provided with instructions for use, a list of indications and contraindications for use. Manufacture Rep, Almagia International, provides quality service and maintenance, and provides a full guarantee on all products. A Transcranial magnetic stimulation device.
Stabilizes vision in patients with chronic iridocyclitis;
Relieves pain in patients with migraine;
Effective in the acute and subacute period of stroke;
Improves the quality of life of patients with Parkinson’s disease.
| Indications: | Contraindications: |
|
• Osteochondrosis of the cervical spine with symptoms of cranialgia, cephalgia; |
• Bleeding liability |
Specifications:
• AC power supply: ~220V (-10%; +10%) or ~230V (-10%; +6%), frequency 50Hz
• Power consumption:10 VA.
• Overall dimensions:
Control unit - 210x170x75 mm;
Flexible emitting line- 300x50 x30 mm.
• Field types:
“travelling”, when there is a serial actuation of all the emitters of the flexible emitting lines from the 1st emitter to the 6th in cycles (pro-grams No.1, No.2, No.3);
“static”, when there is a simultaneous actuation of all the emitters (program No.4).
• The frequency of emitter excitation with single pulses:
pulses / sec (program No.2)
Relative deviation of the frequency rate is within ±5%.
• The frequency of emitter excitation with pulse packets changes automatically as follows:
from 1 to 5 pulses / sec (programs No.1, No.4)
from 5 to 15 pulses / sec (program No.3);
Relative deviation of the frequency rate extremes is within ±10%.
• The time during which the repetition frequency of pulse packets changes from maximal to minimal value and back is: 126±10 sec for program No.3 and 612±30 sec for programs 1 and 4;
• Pulse frequency within each packet:
30 pulses / sec (program No.3);
7 pulses / sec (programs No.1, No.4);
Relative deviation of the frequency value is within ±5%;
• Peak values of the magnetic field density on the emitters’ surface are:
10 mT (programs No.1, No.2, No.3);
8 mT (program No.4);
Absolute deviation of the field density peak value (B) is within ±[0.2B+0.6] mT.
• The device provides storage of 4 preset exposure programs in its non-volatile memory.
• The non-volatile memory of the device allows storage and dis-play (after plugging-in) of the last used program number.
• The surface temperature of the emitters of the flexible emitting lines does not exceed 41 ºС.
• The setting time of the device operating mode does not exceed 60 s.
• The flexible emitting lines contain labeling of the magnetic field pole: “N” = north.
• In case of the device malfunction, generation of alarm signaling and automatic termination of the exposure mode is provided.
• The device displays the following indications:
program number;
exposure time;
presence of magneto-action.
• The duration of the device continuous operation is at least 8 hours in an intermittent cycle: 20 minutes of magneto-action are fol-lowed by a 5-minute break.
• Mean lifetime - 5 years.
• The exterior surfaces of the device components are resistant to disinfection with any chemical solution approved in medical practice for application for plastic and metal products.
Manual in English and Russian languages
Related Products
Perforated training glasses are designed and manufactured by LLC...
Device «EHF-ND 01» is intended to treat a wide spectrum of diseases,...
Prostate physiotherapy device can aid in the recovery of the prostate...

All manufacturer names, symbols, and descriptions, used in our images and text are used solely for identification purposes only.