Best Sellers
To treat what
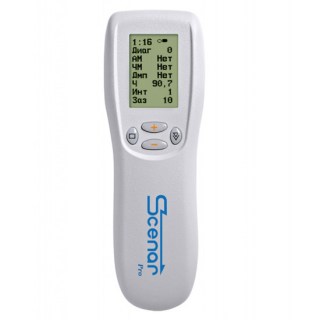
SCENAR1-NT v.02.2 OPTIMA
Welcome to SCENAR Technology! This is the only original Scenar device in the world with CE certification.
The Professional version has digital readings to find the exact locations for treatment and easily compares the various reactions of the patient's body during treatment. The built-in biofeedback protocol exactly indicates when the optimum stimulation on a specific place has been reached.
The Scenar signal used can be standard or, if required, can be modified by manually setting one or more of the five variables or by choosing one of the many predefined signals. It is has a built in microprocessor and the software can be updated in the future with new functions.
Results of SCENAR therapy: During 30 years of Scenar development, Universities and medical specialists have focused on the following specific areas:
- Bronchi pulmonary system
- Cardiovascular system
- Nervous system
- Gastro enteric system
- Uro-genital system
- Endocrine glandular system
- Immune system
- Bones-muscles-ligament system
- Dermal system
- Emergency trauma treatment
- Pain management
For each of these areas, the contribution of Scenar therapy for health recovery has been defined and tested. This has resulted in specific treatment schemes and protocols for those areas as well as generic, non-specific treatment schemes that are applied to treat the body in an integrated way.
Model SCENAR-1-NT (version 02.2) (also known as Scenar Optima) represents optimal combination of price and quality. Compared to the previous model (SCENAR-1 (version 02.3)), the functional unit is extended by addition of the second dosed mode (”Diag 2” - the differential dose) and a combined modulation mode (Swing) which enables you to change shape and gap between pulses and frequency of their repetition. The device is most popular among sports medical professionals and manual therapists.
Full manual in English and Russian!
The RITMSCENAR Pro device supports two Modes of operation:
Manual Mode –all device settings, localization of active points and treatment durations are determined subjectively by the therapist.
Dosing Mode – Dose1 –– treatment of a particular skin area is dosed automatically by the device. The treatment time is determined by the device and depends on the changes in skin parameters.
The device can be set up with various amplitude modulations depending on the stage of the pain process, treatment area and the individual response of the body. The Amplitude modulations integrated into the RITMSCENAR Pro are: 1:1, 1:2, 1:3, 1:4 and 1:5.
Five Damping modes are available in the RITM SCENAR Pro to be used according to the complaint phase, skin sensitivity and body response.
The Frequency Modulation incorporated in the RITM SCENAR Pro is from 30Hz to 120Hz.
Influencing Frequencies can be selected within the range of 15Hz to 350Hz and can be utilized in the treatment of different phases of the complaint.
The practitioner can also control and modify other parameters of the device, such as: impulse intensity, duration of gaps between impulses and power influence for achieving optimal treatment results.
| Indications | Contraindications |
| RITMSCENAR Pro is a non-invasive, electrotherapeutic, hand-held device. It is intended to be used as an analgesic peripheral nerve stimulator, to be placed on the skin to treat pain associated with: Surgery Trauma Musculoskeletal problems Bursitis Dental problems The RITMSCENAR Pro facilitates functional restoration and improvement and is suitable for physical therapy and during labour/delivery. |
Do not use SCENAR if you have any of the following conditions: Individual intolerance (hyper sensitization); Heart pacemaker; Serious mental diseases; Pregnancy; Self-help in case of alcohol intoxication; Acute infectious diseases of obscure origin. |
Technical data of Scenar models:

Certifications:
USA (FDA code of Federal Regulations): The SCENAR device, Biofeedback-controlled electro-stimulator, is regulated by the United States Food and Drug Administration, under 21 CFR 882.5050, Generic Name: Device, Biofeedback, Product Code HCC, Class II exempt from 510(k) pre-market notification according to FDA. Notice (Federal Register), Jan. 21, 1998, p. 84) as a battery-powered, professional use device . http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?FR=882.5050
EUROPE : The SCENAR device has received a CE-mark in Europe (CE 60906 BSI).
AUSTRALIA : The SCENAR device listed on the Australian Register of Australian Goods (ARTG). The registration listing number is 101783
Please note that Scenar requires 1x AAA Batteries. I can`t put it in the package because it is forbidden for airmail.
Related Products

All manufacturer names, symbols, and descriptions, used in our images and text are used solely for identification purposes only.