Best Sellers
To treat what
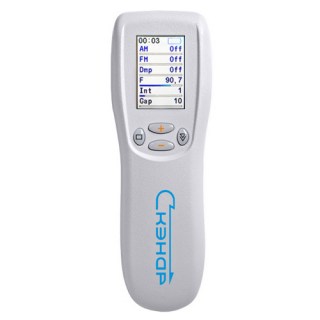
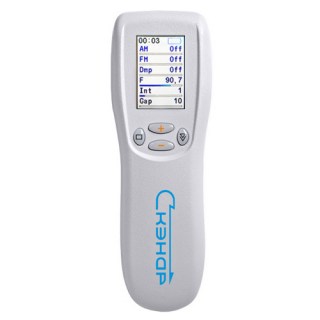
SCENAR1-NT SUPER PRO (BioScenar)
Welcome to SCENAR Technology! This is the only original Scenar device in the world with CE certification.The Ritm SCENAR SUPER Pro is a hand-held, therapeutic medical device licensed use to alleviate pain and facilitate functional restoration and improvement.
The Ritm SCENAR SUPER Pro is the newest development in SCENAR technology by RITM OKB and its new FLAGSHIP!
Ritm SCENAR Professional series of devices are designed for use by medical and health care practitioners such as Physiotherapists, Chiropractors, Nurses, Osteopaths, Rehabilitative Therapists, Massage Therapists and Sports Medicine Therapists to complement their treatments or to provide specialist Ritm SCENAR therapy.
The Ritm SCENAR SUPER Pro uses a sophisticated software program which controls and automatically changes the impulse waveforms and frequencies to prevent the body from adapting to the stimulation.
The Ritm SCENAR SUPER Pro series measures skin impedance and displays the information on an LCD screen. This allows the medical practitioner to the most appropriate mode of operation and zone of treatment.
As compared with the previous version of the device (RITMSCENAR Pro Plus), its functionality is extended to include the third mode of screening (Dose 5) — screening with the external electrodes using REFLEXODIAGNOSTICS by NAKATANI.
The most important ’know-how’ of the device is the mode of double biofeedback that allows determining necessary stimulation parameters in acute states in the automatic mode.
The device includes factory pre-settings (presets) to be applied in:
- pain relief,
- cosmetology,
- massage therapy,
- stimulation before and after athletic training,
- post-traumatic recovery and rehabilitation.
Thanks to the built-in presets, even a home user without SCENAR therapy experience can obtain good results after the first sessions. Besides, the device has a color display and new user-friendly interface. The updated menu with fast navigation functions allows to drastically cut the time of configuring the device before use.
It is supplied complete with the external electrodes used for screening in Dose 5 mode.
New functions and treatment protocols are incorporated into the RITMSCENAR Super Pro for optimizing treatment time, expanding treatment applications and delivering more effective and reliable therapy.
RITMSCENAR Super Pro is a light-weight, portable, easy to use, multi-functional and powerful SCENAR device designed for advanced users to provide non-invasive, specialist SCENAR therapy interactive electrical stimulation via the patient’s skin.
SCENAR technology has been proven to provide quick and sustainable pain relief in a wide range of painful conditions, increased function, and quicker rehabilitation.
Purchase includes:
Manual in Russian and English
Ritm SCENAR SUPER PRO Technical Features:
- Diagnostic Mode Integral dose and null
- Dose indication sound and symbolic
- Damping 4 constant modes, 1 variable mode
- Amplitude modulation 1 : 1, 2 : 1, 3 : 1, 4 : 1, 5 : 1
- Frequency modulation 30 - 120 Hz, cycle 7 sec
- Influencing impulse frequencies 0,674-500 Hz
- Intensity (the number of impulses in a packet) 1 to 8
- The duration of gaps between impulses in a packet Z 200 to 1600 sec
- Amplitude of the stimulating pulse at a standard load (1,7-2,5) to (100-150) V
- Indicator Graphic LCD with backlight
- Number of personal sets of parameters Up to 5
Power supply three 1.5V alkaline batteries
Temperature 10 to 35C (Operating) -50 to 50C (Storage & Transportation)
Weight 0.4kg
Dimensions 190 x 70 x 40mm
Certifications:
USA (FDA code of Federal Regulations): The SCENAR device, Biofeedback-controlled electro-stimulator, is regulated by the United States Food and Drug Administration, under 21 CFR 882.5050, Generic Name: Device, Biofeedback, Product Code HCC, Class II exempt from 510(k) pre-market notification according to FDA. Notice (Federal Register), Jan. 21, 1998, p. 84) as a battery-powered, professional use device . http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?FR=882.5050
EUROPE : The SCENAR device has received a CE-mark in Europe (CE 60906 BSI).
AUSTRALIA : The SCENAR device listed on the Australian Register of Australian Goods (ARTG). The registration listing number is 101783
Please note that Scenar requires 4x AAA Batteries. I can`t put it in the package because it is forbidden for airmail.
Related Products

All manufacturer names, symbols, and descriptions, used in our images and text are used solely for identification purposes only.