Best Sellers
To treat what
ESTER
According to various estimates, from 20% to 30% of people with depression do not respond to traditional methods of treatment (drug therapy, psychotherapy, light therapy, etc.), therefore, in these cases, more radical methods are used. First of all, this is electroconvulsive therapy (ECT), which began to be used in the thirties of the twentieth century.
For many diseases, ECT remains the most effective and safe therapy. To date, according to foreign psychiatrists, ECT is ideally suited 80% of patients. It is widely used to treat mental, narcological and neurological diseases.
The device is intended for electroconvulsive (electroconvulsive) therapy in a number of mental, narcological and neurological diseases with a series of unipolar rectangular current pulses passing through the corresponding brain structures and affecting its neurons. An electrical impulse that causes a therapeutic epileptiform seizure in the brain stimulates the hypothalamus and structures of the limbic system, increasing the secretion of peptides (for example, the hypothetical eutimesin peptide) involved in mood regulation. In this case, a fairly rapid decrease in the vegetative manifestations of depression occurs.
The device allows you applying a series of unipolar rectangular current pulses to the brain structures using special electrodes installed monolaterally, bilaterally (bifrontally) on the patient’s head.
It can be used in psychiatric clinics and hospitals.
Indications for use
Schizophrenia.
Bipolar affective disorder.
Parkinson's disease.
Severe depression, resistant to psychotropic drugs:
1) acute depression with severe anxiety, fears, rapidly increasing physical exhaustion, serious suicidal tendencies, catatonia;
2) protracted depression with the monotony of affective manifestations, delusional ideas of sinfulness, hypochondriacal delirium, delirium of nihilistic content, verbal hallucinations.
Atypical psychoses.
Febrile schizophrenia.
Acute catatonic agitation or catatonic stupor.
Epilepsy.
Severe diseases of the cardiovascular system - severe myocardial changes, decompensated heart defects, angina pectoris, coronary sclerosis, severe general atherosclerosis, stage II and III hypertension, thrombophlebitis.
Diseases of the musculoskeletal system with a risk of fractures: deforming arthritis, poorly healed fractures, osteomyelitis, severe kyphoscoliosis, osteoporosis, limited mobility of joints of traumatic or inflammatory origin.
Organic diseases of the central and peripheral nervous system (parkinsonism, multiple sclerosis, etc.).
Acute and chronic infections, purulent diseases.
Acute bronchitis, bronchiectasis, pulmonary emphysema, bronchial asthma.
Acute and chronic diseases of the nasopharynx with impaired nasal obstruction.
Peptic ulcer of the stomach and duodenum.
Diseases of the liver and kidneys; diabetes; hyperthyroidism; retinal detachment; pregnancy.
Relative contraindications
Hypertension stage I.
Moderate atherosclerosis.
Compensated heart defects.
Femoral and inguinal hernias.
Well-fused old fractures.
Specifications:
The maximum value of the amount of electricity (dose) given per procedure - 330mCl
Dose range - 30-330mCl
Dose setting resolution - 1mCl
Type of impulses - a series of unipolar rectangular pulses
Pulse Series Character - continuous and modulated - 5 pulses / pause / 5 pulses
Pulse Series Trigger Mode
- with a set amplitude;
- with a smooth increase in amplitude for 1 second to the set
Amplitude of current pulses - 550mA and 850mA
Active load - no more than 400 Ohm
Current pulse duration - 0.5ms; 1ms and 1.5ms
Pulse repetition rates - 27Hz; 40Hz; 60Hz and 77Hz
Mode of procedure - manual and automatic
Machine Ready Time- no more than 10 sec
Device operating mode - intermittent:
2 hours - work
15 min - pause
Type of indication of procedure parameters - liquid crystal display
Test mode - automatic when turning on the device and manual if necessary
Number of treatment electrodes - 2 pcs.
Dimensions of the electronic unit - 360x280x165mm
Mass of the electronic unit - no more than 5 kg
Power consumed by the device from an alternating current network (220 ± 22V), frequency 50Hz - no more than 50V
The device complies with GOST R 50267.0-92 and is made in terms of electrical safety, as a class I product of type B.
For its operation, it is necessary to have a power outlet that has a third contact connected to the ground loop (Euro outlet).
Contents of delivery:
1. The electronic unit of the ESTER apparatus with procedural electrodes - 1 pc.
2. The gel is electrically conductive- 1 pc.
3. Mains power cable- 1 pc.
Passport combined with technical description and operating instructions - 1 pc.
Related Products
Perforated training glasses are designed and manufactured by LLC...
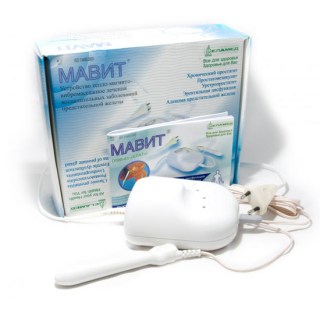
Prostate physiotherapy device can aid in the recovery of the prostate...

All manufacturer names, symbols, and descriptions, used in our images and text are used solely for identification purposes only.